Out Of This World Tips About How To Manage Clinical Trials

Streamline operational workflows at your clinical trial site.
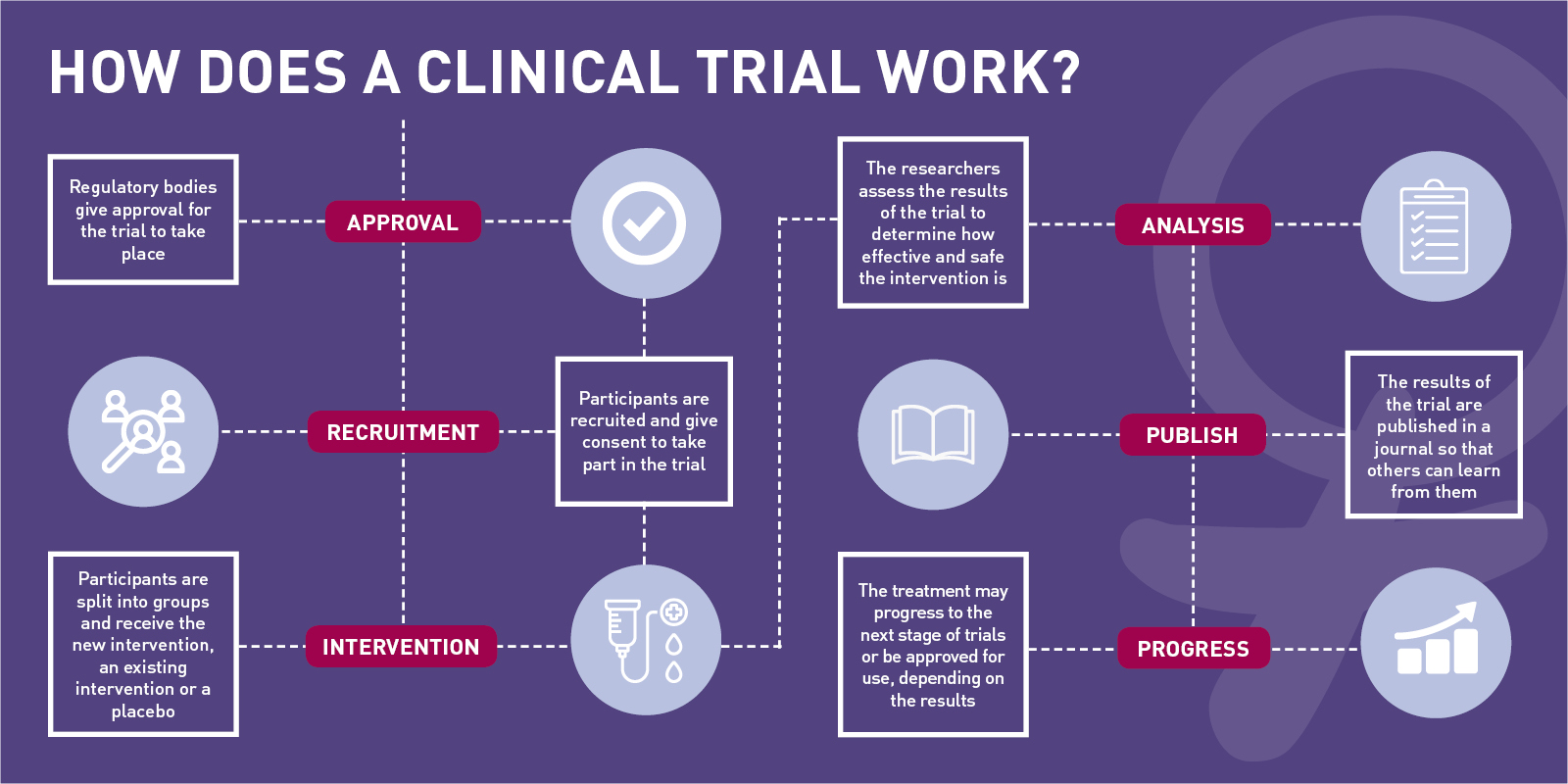
How to manage clinical trials. What are the types of clinical trials? Clinical trials are conventionally monitored by source data verification that is costly, requires ample resources, and exhibits several limitations. 1) unify datasets to capture a complete view of patient and study data.
Workload tip 1: The european commission, the european medicines agency (ema) and national head of medicines agencies (hma) have published new recommendations for. Guideline outlines principles for good clinical practice and trials management that apply to all trials of clinical and public health interventions.
Learn strategies to beat challenges that hurt clinical trial management speed. Currently, clinical trial data is captured in siloed systems. This software has modules for project.
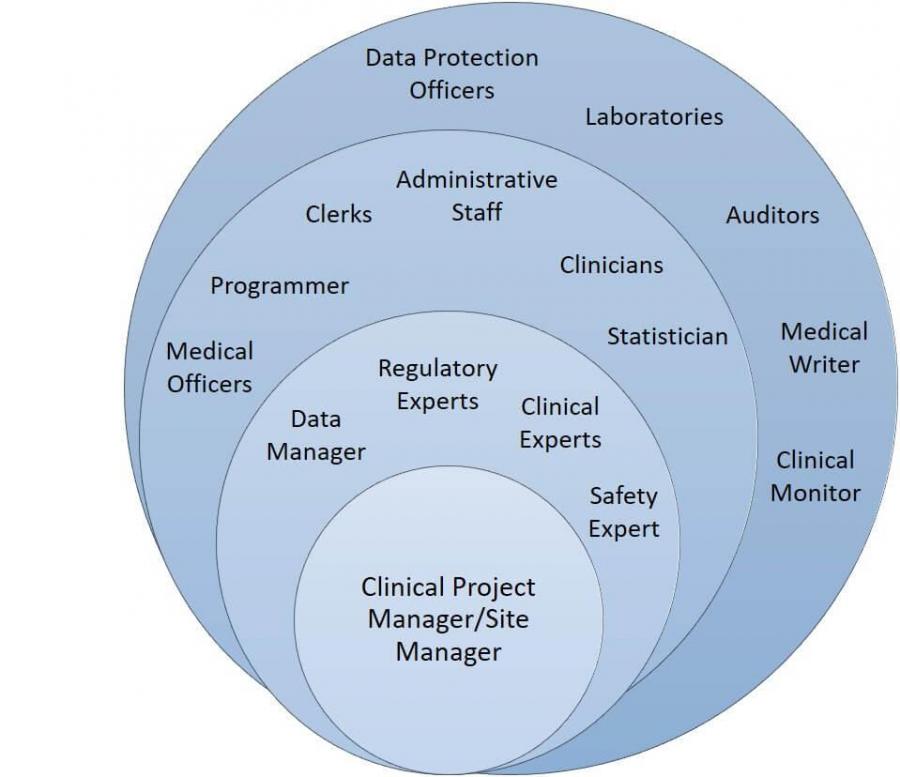
A clear objective aimed to bring about change 2. Clinical trials play a central role in determining how medicine is practiced [ 1 ]. All sites have formal and informal sops (standard of practices).
A clinical trial management system (ctms) is software specifically designed for the unique needs of clinical trials. Clinicaltrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. This presentation is only complementary to the guidance which.
Clinical trials management and advanced operations. Get best practices to manage costs. The clinical trial process is critical for regulatory approval of new medical interventions and the establishment of new standards of care.
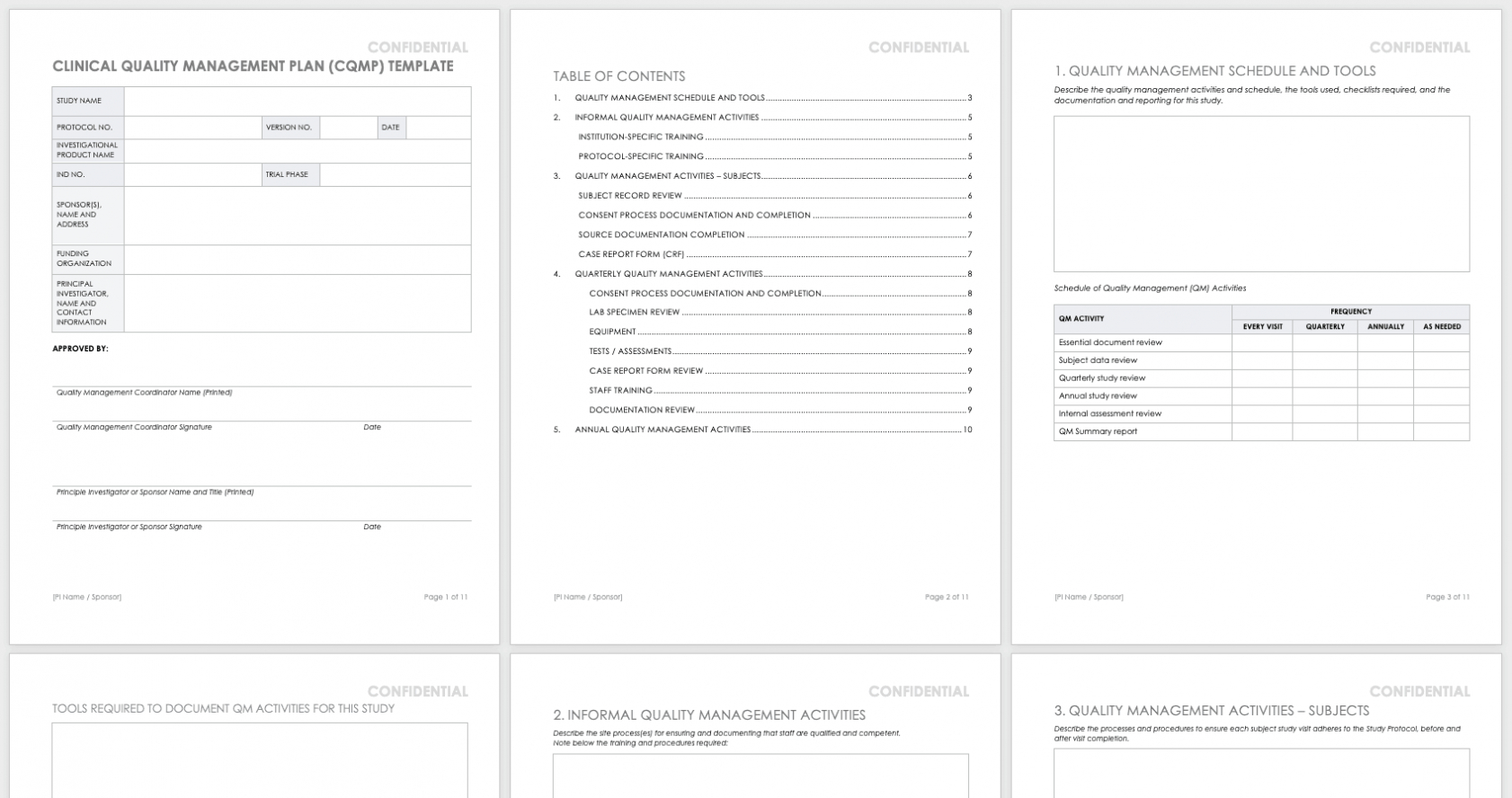
A set time scale 4. What do the terms placebo, randomization, and blinded mean. 15+ years managing data—and change—in clinical trials, we’ve created this data management playbook to help you tackle change when the time comes.
Clinical trials in the uk. What are the phases of clinical trials? Veeva econsent provides a digital way to consent clinical trial participants in person or remotely.
This course is part of clinical trials operations specialization. What is informed consent? Defined resources to achieve its objective 5.
Postgraduate medical journal, volume 96, issue 1139,. The trial will be conducted in accordance with the principles of the declaration of helsinki and approved by tohoku certified review board of tohoku university. National attention has focused on engaging patients in the.